Measuring the quality of water
Measurement of the acidity/basicity of solutions, of the concentration of a substance in solutions, is done using various types of probes, such as the pH or Redox probes. These probes have various types of outlets, which are mostly current loops or voltage outputs. It is recommended to use the C-IT-0200I module to process the signal from these probes, as it is suitable both for the measurement of the current loop and the voltage output of the probes. In the case of the current loop, the probe is equipped with an output that converts the measured value into the range of 0 to 20 mA or 4 to 20 mA.
In the case of the voltage output, it depends whether the output of the probe can convert the measured values into a standard range, such as 0 to 10 V, or whether the probe only has a direct output.
In the case of a direct voltage output, the following ranges are used for measurements by the C-IT-0200I module: "HI -1V ÷ 1V" and "HI -100mV ÷ 100mV". In this case, the problem with the pH and Redox measurements is their high internal resistance. The C-IT-0200I module input then does not measure the open circuit voltage, but the voltage reduced by the decrease in the internal resistance of the probe. A necessity that arises from this fact is the calibration of values of the measured data. Calibration of both types of probes is done using calibration solutions. First, dip the probe into the solution with a known value of the measured variable, and subtract the corresponding voltage. Then repeat this procedure with different values of the measured variable. In this way you obtain a set of values that can express the transfer characteristics of the sensor. The measured value of the variable can then be calculated from the obtained relation. The calibration is not usually done in the whole range of the probe, but just for a few values, since the most common application is monitoring whether the limit value of the concentration of the substance or its pH have been exceeded. Therefore, you only need to know the values around the selected limit concentration of the solution, or its pH.
The pH value represents a measure of neutrality, acidity or pollution of the aqueous solution.
Pure water is neutral and its pH is 7. 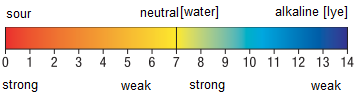
Everything below this value is referred to as acid, everything above this value is alcalic.
